More than half a million bottles of blood pressure medication are being recalled over a cancer-causing chemical connected to the prescription drug, according to the U.S. Food and Drug Administration.
Teva Pharmaceuticals USA, based in Parsippany, New Jersey, issued a voluntary recall on Oct. 7 for some of the prazosin hydrochloride capsules it distributed, and the FDA classified it as a Class II risk level on Friday, Oct. 24.
The drug was approved by the FDA to treat high blood pressure, but sometimes prescribed off-label to help manage symptoms of post-traumatic stress disorder (PTSD), particularly nightmares and sleep problems. The medication works by relaxing blood vessels, improving blood flow and reducing blood pressure.
More: Neutrogena recalls makeup wipes after they test positive for bacteria
According to the FDA, a Class II risk is a situation “in which use of or exposure to a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote.”
More: Taco dinner kits sold at Aldi, more recalled over seasoning mix-up
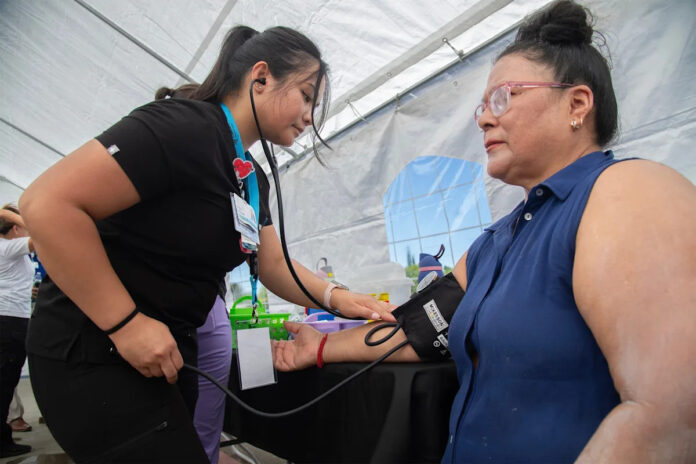
Nurse Haley Delgado, left, takes Maria Hernandez’s blood pressure at the Community Medical Center’s Health and Resource Fair at Banner Island Ballpark in downtown Stockton, California, on Aug. 9, 2025.
The risk classification from the federal agency indicates the recalled medication may contain nitrosamine impurities also called “N-nitroso Prazosin impurity C.” Exposure to the product can lead to severe health risks, the FDA reported.
Here’s what to know about the recall, including how many bottles have been recalled.
More: Eggs, cinnamon, chicken, pork. See recent food recalls you should know about.
An elderly patient has her blood pressure taken by a nurse.
What blood pressure medicine is being recalled?
The recall involves more than 580,000 prazosin hydrochloride capsules distributed by Teva Pharmaceuticals.
The prescription affects:
-
1 mg capsules: 181,659 bottles
-
2 mg capsules: 291,512 bottles
-
5 mg capsules: 107,673 bottles
The bottles may contain anywhere from 100 to 1,000 capsules, according to the FDA.
For more information about the Code Information and the recall lot number, visit the FDA’s Enforcement Report here.
What should people do with recalled medication?
Neither Teva nor the FDA issued guidance on what to do with the recalled tablets.
But according to GoodRx, anyone affected by a drug recall is advised to check their medication’s lot number, contact their pharmacist as well as their prescriber and throw away the recalled medication.
USA TODAY has reached out to Teva.
Contributing: USA TODAY’s James Powel.
Natalie Neysa Alund is a senior reporter for USA TODAY. Reach her at nalund@usatoday.com and follow her on X @nataliealund.
This article originally appeared on USA TODAY: Blood pressure medicine recalled over cancer risk